Produktion von Lentiviren mit METAFECTENE®
Lesen Sie hier einen Anwendungsbericht für die Produktion von Lentiviren. Für die Transfektion der Zellen (293T/17) wurde das Transfektionsreagenz METAFECTENE® verwendet. Nach 2 Tagen Inkubationszeit lag die Transfektionseffizienz bei nahezu 100%.
Metafectene® technical note for lentiviral production
Adriana Grbenicek1, Bernhard Berkus2, Patrick Maier1
1Department of Radiation Oncology, University Medical Center Mannheim, Heidelberg University, Germany
2DKFZ (German Cancer Research Centre), Heidelberg, Germany
Virus production: Transfection of 293T/17 cells for production of lentiviruses
The here described procedure for production of lentiviruses extends over four days plus three more days for determining the viral titer as described in [1].
Material:
Plasmid pHR’SINcPPT-SEW carrying EGFP as transgene controlled by the SFFV promoter
Plasmid pCMVDR8.91 packaging plasmid containing the HIV genes gag and pol
Plasmid pMD.G encoding the envelope protein VSV-G
Metafectene: Biontex T020-5
Na-butyrat: Sigma Aldrich B-5887;1 M stock solution in PBS
Poly-D-Lysine: Sigma P-7280; 1 mg/ml stock solution in PBS
DMEM with FCS and P/S
DMEM without additives
Millex-Ha Filter 0.45 µm: Millipore SLHA 033SS
Vivaspin 100 000 MW cut-off: Sartorius VS2041
The lentiviral vector system
The lentiviral plasmid pHR’SINcPPT-SEW [2] contains the information of the recombinant lentiviral genome which is devoid of any information for the original HIV proteins. Instead, flanked by the 5’LTR (long terminal repeat domain) and the D3’LTR (with deleted promoter sequences in order to reduce the risk of activation of downstream genes after integration into the human genome) the transgene EGFP (enhanced green fluorescent protein) is placed under the control of the U3 region of the LTR of SFFV (spleen focus-forming virus) and upstream of the WPRE (woodchuck hepatitis virus posttranscriptional regulatory element). An additional element is the packaging signal Y which is required that the viral RNA will be used as viral genome and packed into the viral capsid. The capsid protein and the reverse transcriptase are encoded by the genes gag and pol on the packaging plasmid pCMVDR8.91. The third plasmid delivers the information for the envelope protein VSV-G (vesicular stomatitis virus glycoprotein) for pseudotyping (enlargement of the spectrum of transducable cell types even of different species) of the viral capsid. This is a packaging system of the second generation (for further information about lentiviral vector systems see [3]).
Protocol
All information is for a culture dish of a diameter of 10 cm, and all solutions should be warmed up to room temperature.
Day 1: The culture dish is coated with 3 ml Poly-D-Lysine (0.1 mg/ml in PBS). After 5 min, Poly-D-Lysine is removed and stored at 4 °C since it can be used several times. The dish is washed with 5 ml sterile water and thus ready for use. 5 x 106 293T/17 cells are seeded in 14 ml DMEM with additives and cultured overnight.
Day 2: On the second day transfection is performed with three plasmids.
Overall 10 µg of DNA is used for one culture dish: 4.4 µg lentiviral plasmid, 3.4 µg pCMVDR8.91, and 2.2 µg pMD.G. The plasmids are given into a well of a 24-wellplate and mixed thoroughly with 700 µl DMEM without additives à solution A.
46 µl Metafectene is mixed in another well of a 24-wellplate with 700 µl of DMEM without additives à solution B
Then solution A and B are mixed in one well and incubated for 20 min at room temperature. The solution of nearly 1.5 ml is added drop by drop to the cells with concomitant gentle orbital shaking of the dish. This is followed by an overnight incubation at 37 °C.
Day 3: In the morning, the medium is replaced by 14 ml DMEM with additives and 140 µl Na-butyrate (10 mM).
In the evening the medium is replaced by 7 ml DMEM without additives.
Day 4: The medium is collected (it can be stored at 4 °C for several hours) and filtered with a 0.45 µm filter. The cleared virus-containing solution is put on a Vivaspin-tube and concentrated up to 0.5 ml or 1 ml final volume (depending on the number of 10 cm dishes used for virus production) with 3000 rpm (≙ ca. 1800g) at 4 °C in an Eppendorf tabletop centrifuge. Aliquots of 50 µl or 100 µl are stored at -70 °C.
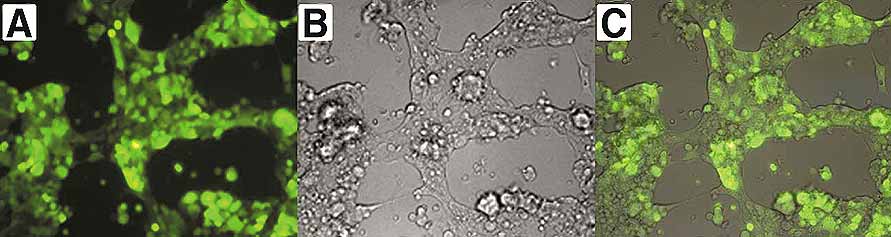
Figure 1. EGFP-expressing cells two days after transfection. Overlay (C) of the fluorescent channel (A) with the bright field picture (B) illustrates that a nearly 100 % transfection efficiency was achieved with the transfection conditions as described in our protocol.
Virus titration: Transduction of HT1080 cells
Material:
24 well plate
polybrene hexadimethrine bromide: Sigma H-9268
polybrene working solution: 800 µg/ml PBS sterile filtrated, storage at 4°C for months
PBS without Ca/Mg: Gibco 14190-094
DMEM: Gibco 41965-039
Protocol
All work must be done under S2 conditions.
Day 1: 8x104 HT 1080 cells are seeded per well of a24 well plate. Titration of each dilution should be done in duplicates or triplicates!
Day 2: Four dilutions are analysed: 1:100, 1:1000, 1:10000, 1:100000:
1. dilution (1:100): 10 µl viral supernatant + 980 µl medium + 10 µl Polybrene working solution
further dilutions: 100 µl of the previous dilution + 890 µl Medium + 10 µl Polybrene working solution
The medium on cells is replaced by 300 µl of each dilution.
Day 3: The medium is exchanged with 0.5 ml DMEM without Polybrene per well
Day 4 or 5: The cells are harvested, washed once with 1 ml PBS (centrifuge 3 min with 3000 rpm ≙ ca. 1800g in an Eppendorf tabletop centrifuge) and 0.3-1 x106 cells are resuspended in 300 µl staining medium for FACS.
Analysis
Example: 80000 cells ≙ 100 %
FACS result: 22.5 % GFP-positive cells 22.5 % transduction efficiency
supernatant diluted 1:1000 in 300 µl
=> amount of viral particles in 1 ml concentrated supernatant = (transduction efficiency x total cell number)/100 % x (1 ml/volume of medium containing diluted viral particles) x dilution factor
= (22.5 % x 80000) / 100 % x (1000 µl/300 µl) x 1000
= 22.5 x 8000/3 x 1000
= 6 x107 infectious particles (titer) per 1 ml
References:
- Maier, P.; Herskind, C.; Fleckenstein, K.; Spier, I.; Laufs, S.; Zeller, W. J.; Fruehauf, S.; Wenz, F., MDR1 gene transfer using a lentiviral SIN vector confers radioprotection to human CD34+ hematopoietic progenitor cells. Radiat Res 2008, 169, (3), 301-10.
- Demaison, C.; Parsley, K.; Brouns, G.; Scherr, M.; Battmer, K.; Kinnon, C.; Grez, M.; Thrasher, A. J., High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 2002, 13, (7), 803-13.
- Maier, P.; von Kalle, C.; Laufs, S., Retroviral vectors for gene therapy. Future Microbiol 2010, 5, 1507-23.